An Open-labelled, Prospective Clinical Trial to Evaluate the Safety & Efficacy of Alsarex in the Treatment of Acid Peptic Disorders
DOI:
https://doi.org/10.21760/jaims.10.7.4Keywords:
Alsarex Tablet, Acid Peptic Disorders, Gastrointestinal Symptom Rating Scale (GSRS), dyspepsia, gastric ulcer, duodenal ulcer, Zollinger-Ellison syndrome, functional dyspepsiaAbstract
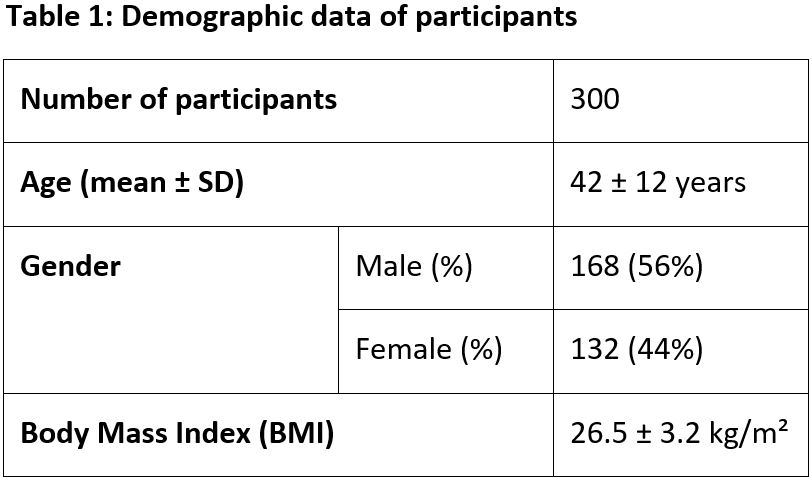
Objectives: To evaluate the clinical efficacy and safety of Alsarex Tablets in Acid Peptic Disorders. Material and Methods: A prospective, open-label clinical study was conducted on 300 patients of both sexes, aged between 18-65 years, confirmed with Acid Peptic Disorders from clinical examination, laboratory tests and who were willing to give informed consent. All patients received Alsarex Tablets at a dose of 1 tablet twice daily half hour before meals for 4 weeks. All patients were evaluated at baseline and 4 weeks using the Gastrointestinal Symptom Rating Scale (GSRS), a validated and standardized tool for assessing symptoms of acid peptic disorders. Observation: After 4 weeks of treatment, Alsarex Tablets showed significant reduction across all evaluated symptoms in the Gastrointestinal Symptom Rating Scale (GSRS); with p-values less than 0.001, indicating statistically significant reductions in symptom severity after treatment. The greatest improvements were noted in symptoms such as nausea, rumbling in the stomach, and loose stools, where the mean severity scores decreased notably from baseline values. Alsarex showed a broad therapeutic effect across diverse gastrointestinal symptoms, including pain or discomfort in the upper abdomen, heartburn, acid reflux, bloating, burping, and bowel movement irregularities. Result: Alsarex Tablets were found to be effective and safe in controlling the signs and symptoms of acid peptic disorders and its associated complications. There were no clinically significant adverse events either reported or observed during the entire study period. The overall compliance with the treatment was good and no treatment discontinuations were reported.
Downloads
References
Chavan MS, Bhaktavatsalam M. Prevalence and risk factors of acid peptic ulcer disease at a tertiary care hospital. Int J Adv Med. 2018;5(4):988–992. doi:10.18203/2349-3933.ijam20183134
Taraszewska A. Risk factors for gastroesophageal reflux disease symptoms related to lifestyle and diet. Rocz Panstw Zakl Hig. 2021;72(1):21–28. doi:10.32394/rpzh.2021.0145. PMID: 33882662
Yadav R, Kumar J, Singh AK, Yadav A, Gupta PC. A review on the pathogenesis, treatment and prevention of peptic ulcer disease. Asian J Med Princ Clin Pract. 2022;5(4):183–197.
Kuna L, Jakab J, Smolic R, Raguz-Lucic N, Vcev A, Smolic M. Peptic ulcer disease: a brief review of conventional therapy and herbal treatment options. J Clin Med. 2019;8(2):179. doi:10.3390/jcm8020179
Kumar PV, Palatty PL, Achuthan CR. Current therapeutic approaches in acid peptic disease. J Inflamm Bowel Dis. 2023;8:185.
Meng R, Chen LR, Zhang ML, Cai WK, Yin SJ, Fan YX, et al. Effectiveness and safety of histamine H2 receptor antagonists: an umbrella review of meta-analyses. J Clin Pharm. 2023;63:7–20. doi:10.1002/jcph.2147
Yibirin M, De Oliveira D, Valera R, Plitt AE, Lutgen S. Adverse effects associated with proton pump inhibitor use. Cureus. 2021;13(1):e12759. doi:10.7759/cureus.12759. PMID: 33614352; PMCID: PMC7887997
Lakshmanan M. Drugs used in acid peptic disorders. In: Paul A, Anandabaskar N, Mathaiyan J, Raj GM, editors. Introduction to Basics of Pharmacology and Toxicology. Singapore: Springer; 2021. doi:10.1007/978-981-33-6009-9_34
Yibirin M, De Oliveira D, Valera R, Plitt AE, Lutgen S. Adverse effects associated with proton pump inhibitor use. Cureus. 2021;13(1):e12759. doi:10.7759/cureus.12759. PMID: 33614352; PMCID: PMC7887997
Gupta M, Kapoor B, Gupta R, Singh N. Plants and phytochemicals for treatment of peptic ulcer: an overview. S Afr J Bot. 2021;138:105–114. doi:10.1016/j.sajb.2020.11.030
Saini R, Sharma N, Oladeji OS, Sourirajan A, Dev K, Zengin G, et al. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: a comprehensive review. J Ethnopharmacol. 2022;282:114570. doi:10.1016/j.jep.2021.114570
Chatterjee A, Chattopadhyay S, Bandyopadhyay SK. Biphasic effect of Phyllanthus emblica L. extract on NSAID-induced ulcer: an antioxidative trail weaved with immunomodulatory effect. Evid Based Complement Alternat Med. 2011;2011:146808. doi:10.1155/2011/146808
Pal D, Mukherjee S. Role of amla (Emblica officinalis) in peptic ulcer. In: Herbs, Spices, and Medicinal Plants for Human Gastrointestinal Disorders. 1st ed. 2022. eBook ISBN: 9781003189749
Murray MT. Glycyrrhiza glabra (Licorice). In: Textbook of Natural Medicine. 2020:641–647.e3. doi:10.1016/B978-0-323-43044-9.00085-6. PMCID: PMC7348626
Yadav RK, Nandy BC, Maity S, Sarkar S, Saha S. Phytochemistry, pharmacology, toxicology, and clinical trial of Ficus racemosa. Pharmacogn Rev. 2015;9(17):73–80. doi:10.4103/0973-7847.156356. PMID: 26009696; PMCID: PMC4441165