A Prospective, Open-Label Non-Randomized Clinical Trial to evaluate the safety and efficacy of Pigmento Tablet & Ointment in treatment of Vitiligo
DOI:
https://doi.org/10.21760/jaims.10.7.2Keywords:
Pigmento Tablet, Pigmento Ointment, Vitiligo, Shvitra, AyurvedaAbstract
Background: Vitiligo is a chronic dermatological condition characterized by the loss of skin pigmentation due to the destruction or dysfunction of melanocytes. It can affect individuals of all ages, genders, and ethnicities, often leading to psychological and social distress due to its visible impact on appearance. Recent research into alternative therapies and repurposed treatments has gained momentum, offering potential for improved outcomes. However, well-structured clinical trials are essential to validate these therapies. PIGMENTO Tablet & Ointment, are polyherbal formulations manufactured by Charak Pharma Pvt. Ltd., and were evaluated for its efficacy and safety in patients with vitiligo. A total of 300 patients were included in the study to assess the impact of this treatment approach.
Materials & Methods: This phase 3, non-randomized, prospective, multi-centric, open-label clinical trial aimed to evaluate the clinical efficacy and safety of PIGMENTO Tablet & Ointment in managing vitiligo in patients aged 18–60 years diagnosed with depigmented skin patches.
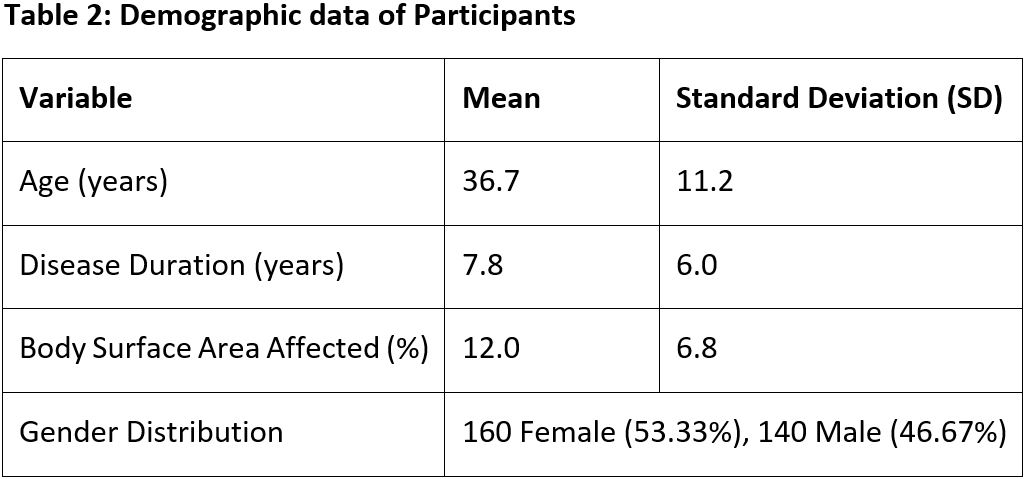
Observation: This clinical trial evaluated the efficacy and safety of Pigmento Tablet and Ointment in 300 vitiligo patients (mean age: 36.7 ± 11.2 years, disease duration: 7.8 ± 6.0 years). The study demonstrated significant re-pigmentation and disease control over three months, with a reduction in affected body surface area (BSA) from 40.2% ± 18.4% to 25.4% ± 15.3% (p = 0.001). Lesion visibility, size, and spread improved notably, alongside a decline in disease activity (p < 0.05). Patients reported relief from symptoms like itching and burning (p = 0.012) and enhanced skin resilience (p = 0.009).
Result: Pigmento Tablet and Ointment demonstrated significant clinical benefits in vitiligo management, including reduced lesion extent, improved appearance, slower disease progression, and enhanced physical and emotional well-being. The therapy was well-tolerated, promoting effective and safe re-pigmentation by stimulating melanogenesis, reducing oxidative stress, and addressing immune-related disruptions. These findings establish Pigmento as a promising and reliable option for improving skin health in vitiligo patients.
Downloads
References
Khoury M, Dabit T, Siniora H, Fashho J, Toubasi AA. The prevalence of vitiligo and its associated risk factors in the Middle East and Africa: A systematic review and meta-analysis. Health Sci Rev. 2024;12:100187. https://doi.org/10.1016/j.hsr.2024.100187
Haulrig MB, Al-Sofi R, Baskaran S, Bergmann MS, Løvendorf M, Dyring-Andersen B, et al. The global epidemiology of vitiligo: a systematic review and meta-analysis of the incidence and prevalence. JEADV Clin Pract. 2024;1:1–10. https://doi.org/10.1002/jvc2.526
National Institute of Arthritis and Musculoskeletal and Skin Diseases. Vitiligo [Internet]. [cited 2025 Jul 24]. Available from: https://www.niams.nih.gov/health-topics/vitiligo
Iannella G, Greco A, Didona D, Didona B, Granata G, Manno A, et al. Vitiligo: Pathogenesis, clinical variants and treatment approaches. Autoimmun Rev. 2016;15(4):335–343. https://doi.org/10.1016/j.autrev.2015.12.006
Spritz RA, Andersen GH. Genetics of vitiligo. Dermatol Clin. 2017;35(2):245–255. https://doi.org/10.1016/j.det.2016.11.013. PMID: 28317533; PMCID: PMC5362127
Cunningham KN, Rosmarin D. Vitiligo treatments: review of current therapeutic modalities and JAK inhibitors. Am J Clin Dermatol. 2023;24:165–186. https://doi.org/10.1007/s40257-022-00752-6
Dhar S, Seth J, Parikh D. Systemic side-effects of topical corticosteroids. Indian J Dermatol. 2014;59(5):460–464. https://doi.org/10.4103/0019-5154.139874. PMID: 25284850; PMCID: PMC4171913
Dodd KC, Ahmed R, Ambrose P, Holt JKL, Jacob S, Leite MI, et al. Mycophenolate and methotrexate are better tolerated than azathioprine in myasthenia gravis. Neuromuscul Disord. 2024;38:51–57. https://doi.org/10.1016/j.nmd.2024.03.010
Speeckaert R, van Geel N. Vitiligo: an update on pathophysiology and treatment options. Am J Clin Dermatol. 2017;18(6):733–744. https://doi.org/10.1007/s40257-017-0298-5. PMID: 28577207
Spritz RA. The genetics of vitiligo: what's new? J Invest Dermatol. 2010;130(5):1313–1315.
Kumar A, Mahendra P, Meena MS. Effect of Bakuchi on vitiligo – a case study. Int Ayur Med J. [Internet]. [cited 2025 Jul 24]. Available from: https://www.iamj.in/posts/images/upload/ISSN23205091.pdf
Kumar A, AlGhamdi KM, Khan AA, Ahamad R, Ghadeer A, Bari A. Psoralea corylifolia (babchi) seeds enhance proliferation of normal human cultured melanocytes: GC–MS profiling and biological investigation. Open Chem. 2023;21(1):20220292. https://doi.org/10.1515/chem-2022-0292
Saxena C, Rawat G. Tinospora cordifolia (Giloy) – Therapeutic uses and importance: A review. Curr Res Pharm Sci. 2019;9:42–45. https://doi.org/10.24092/CRPS.2019.090302
Panwar R, Raj A, Thapa S, Solanki A, Mandola S, Gaurav N. Potential role and assessment of medicinal properties of Giloy (Tinospora cordifolia). Int J Pharmacogn Life Sci. 2023;4(2):12–20. https://doi.org/10.33545/27072827.2023.v4.i2a.86
Singh V, Roy M, Garg N, Kumar A, Arora S, Malik DS. Recent advances in anti-infective drug discovery. Recent Pat Anti-Infect Drug Discov. 2021;16(2):94–121
Bhat PM, Umale H, Lahankar M. Amalaki: A review on functional and pharmacological properties. J Pharmacogn Phytochem. 2019;8(3):4378–4382
Colucci R, Dragoni F, Conti R, Pisaneschi L, Lazzeri L, Moretti S. Antioxidants and vitiligo treatment. Dermatol Ther. 2015;28:17–21. https://doi.org/10.1111/dth.12172