A Prospective, Open-label Non-Randomized Clinical Trial to evaluate the Safety and Efficacy of Calcury Tablets in Treatment of Renal calculi
DOI:
https://doi.org/10.21760/jaims.10.7.6Keywords:
Calcury Tablet, Renal calculi, Kidney stones, Nephrolithiasis, Urolithiasis, Urinary tract obstructionAbstract
Background: Renal calculi, commonly referred to as kidney stones, are a prevalent urological condition characterized by the formation of solid mineral and salt deposits within the urinary tract. The management of renal stones involves a multifaceted approach, including hydration, dietary modifications, pain management, pharmacological interventions, and, in some cases, surgical procedures such as lithotripsy or ureteroscopy. Preventive strategies focus on addressing the underlying metabolic causes and promoting adequate hydration to minimize recurrence.
Materials and Methods: This non-randomized, prospective, open-label clinical trial involving 50 patients aimed to evaluate the clinical efficacy and safety of Calcury Tablets in managing renal calculi in patients aged 20–65 years, a demographic representative of the global population commonly affected by kidney stones.
Study Design: Participants were thoroughly informed about the study procedures, and written consent was obtained before enrollment. The study followed participants over a 4 weeks period to monitor treatment efficacy and safety. Assessments were conducted before and after treatment to evaluate changes in stone size, symptom relief, and recurrence prevention. Any adverse events or complications were carefully documented and analyzed.
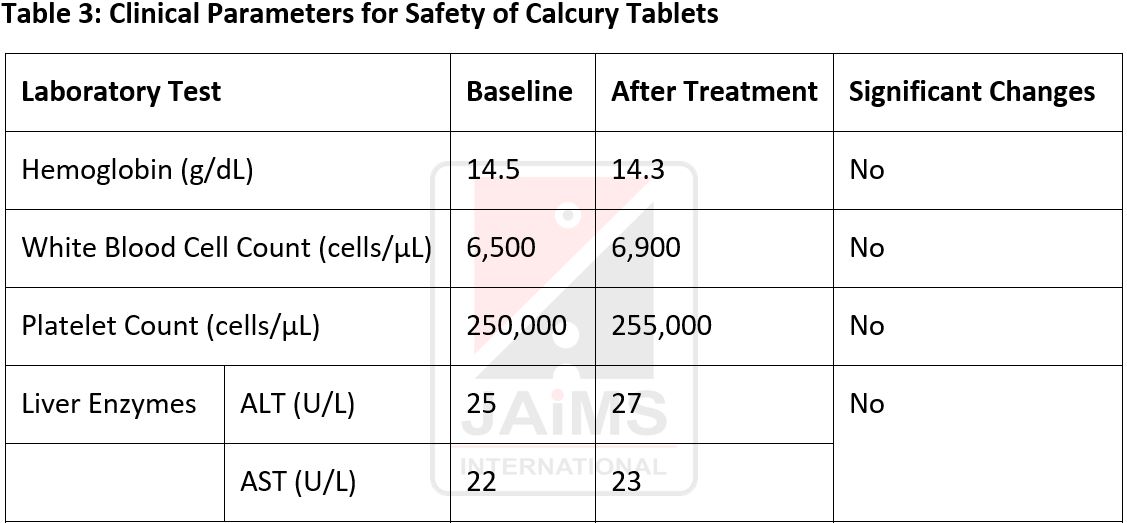
Observations and Results: This clinical trial evaluated the safety and efficacy of Calcury Tablets in managing renal stones in 50 participants (mean age: 47.3 years; BMI: 27.2). Significant reductions were observed in stone size (6.5 ± 1.8 mm to 4.0 ± 1.2 mm, p = 0.001), stone number (3.5 ± 1.4 to 2.1 ± 1.0, p= 0.01), and pain levels (VAS: 6.5 ± 2.0 to 3.2 ± 1.4, p= 0.001). Stone passage increased from 60% to 90% (p = 0.03), and urine pH improved from 5.8 ± 0.6 to 6.3 ± 0.5 (p = 0.05). Quality-of-life scores rose significantly (48.2 ± 8.5 to 65.1 ± 7.2, p = 0.001), alongside hydration improvements (2.0 ± 0.5 to 2.5 ± 0.6 L/day, p = 0.04). Laboratory parameters remained stable, indicating safety, with no significant impact on renal, hepatic, or hematological markers.
Conclusion: The clinical evidence from this trial, combined with the promising preclinical findings on the individual herbs involved in the formulation, supports the use of Calcury Tablets as an effective, safe, and natural alternative for managing renal stones. This treatment offers a promising option for patients seeking a holistic approach compared to conventional medications.
Downloads
References
Leslie SW, Sajjad H, Murphy PB. Renal Calculi, Nephrolithiasis [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan– [updated 2024 Apr 20; cited 2025 Jul 24]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK442014/
Liu Y, Chen Y, Liao B, Luo D, Wang K, Li H, et al. Epidemiology of urolithiasis in Asia. Asian J Urol. 2018 Oct;5(4):205–14. doi: 10.1016/j.ajur.2018.08.007. PMID: 30364478; PMCID: PMC6197415.
Leonard CL. The symptomatology of calculous renal and ureteral disease. JAMA. 1902;39(16):952–5. doi: 10.1001/jama.1902.52480420004001a
Bagga HS, Chi T, Miller J, Stoller ML. New insights into the pathogenesis of renal calculi. Urol Clin North Am. 2013;40(1):1–12. doi: 10.1016/j.ucl.2012.09.006
Soni S, Saboo S, Bhansali A, Barnela S. Medical management of renal stone. Indian J Endocrinol Metab. 2012;16(2):236. doi: 10.4103/2230-8210.93741
National Institute for Health and Care Excellence (NICE). Thiazide diuretics: Evidence review [Internet]. 2019 [cited 2025 Jul 24]. Available from: https://www.nice.org.uk/guidance/ng118/documents/evidence-review-12
Patient.info. Thiazide diuretics [Internet]. [cited 2025 Jul 24]. Available from: https://patient.info/heart-health/high-blood-pressure-hypertension/thiazide-diuretics
Sun Y, Sun H, Zhang Z, Tan F, Qu Y, Lei X, et al. New insight into oxidative stress and inflammatory responses to kidney stones: potential therapeutic strategies with natural active ingredients. Biomed Pharmacother. 2024;179:117333. doi: 10.1016/j.biopha.2024.117333
Haritha C, Ramya D, Naveen R, Prasanna S, Purilla S. A comprehensive review on Bergenia ligulata (Paashanbheda) and its role in the treatment of kidney stone formation. Int J Res Ayurveda Pharm. 2021;12:94–9. doi: 10.7897/2277-4343.1204113
Singh A, Tandon S, Nandi SP, Kaur T, Tandon C. Downregulation of inflammatory mediators by ethanolic extract of Bergenia ligulata (Wall.) in oxalate injured renal epithelial cells. J Ethnopharmacol. 2021;275:114104.
Pareta SK, Patra KC, Mazumder PM, et al. Prophylactic role of Boerhaavia diffusa in ethylene glycol-induced calcium oxalate urolithiasis. Afr J Urol. 2011;17:28–36. doi: 10.1007/s12301-011-0007-1
Arasaratnam V, Sandrasegarampillai B, Senthuran A, Rajendraprasad R. A study of Tribulus terrestris extract on risk factors for urinary stone in normal subjects and urolithic patients. J Natl Sci Found Sri Lanka. 2010;38. doi: 10.4038/jnsfsr.v38i3.2308
Agarwal S, Gupta SJ, Saxena AK, Gupta N, Agarwal S. Urolithic property of Varuna (Crataeva nurvala): an experimental study. Ayu. 2010 Jul;31(3):361–6. doi: 10.4103/0974-8520.77161. PMID: 22131740; PMCID: PMC3221072