A Prospective, Open-label Non-Randomized Clinical Trial to evaluate the Safety and Efficacy of Takzema Tablet & Ointment in Treatment of Eczema
DOI:
https://doi.org/10.21760/jaims.10.6.8Keywords:
Atopic Dermatitis, Eczema, Polyherbal Formulation, Takzema, Clinical Trial, Herbal Medicine, Phase 3 TrialAbstract
Background: Eczema, or atopic dermatitis (AD), is a chronic inflammatory skin disorder characterized by pruritus, erythema, and xerosis, affecting upto 20% of individuals and significantly impacting quality of life. Conventional treatments include topical corticosteroids, calcineurin inhibitors, PDE4 inhibitors, phototherapy, and systemic immunosuppressants, though long-term use may have adverse effects. Advances in translational research have led to targeted small molecules and biologic therapies for moderate-to-severe cases.[1] Takzema Tablet & Ointment, are polyherbal formulations manufactured by Charak Pharma Pvt. Ltd., and were evaluated for its efficacy and safety in patients with Eczema. A total of 300 patients were included in the study to assess the impact of this treatment approach.
Materials and Methods: This phase 3, non-randomized, prospective, open-label, multi-centric clinical trial aimed to evaluate the clinical efficacy and safety of Takzema Tablet & Ointment in managing patients aged 18–60 years diagnosed with Eczema.
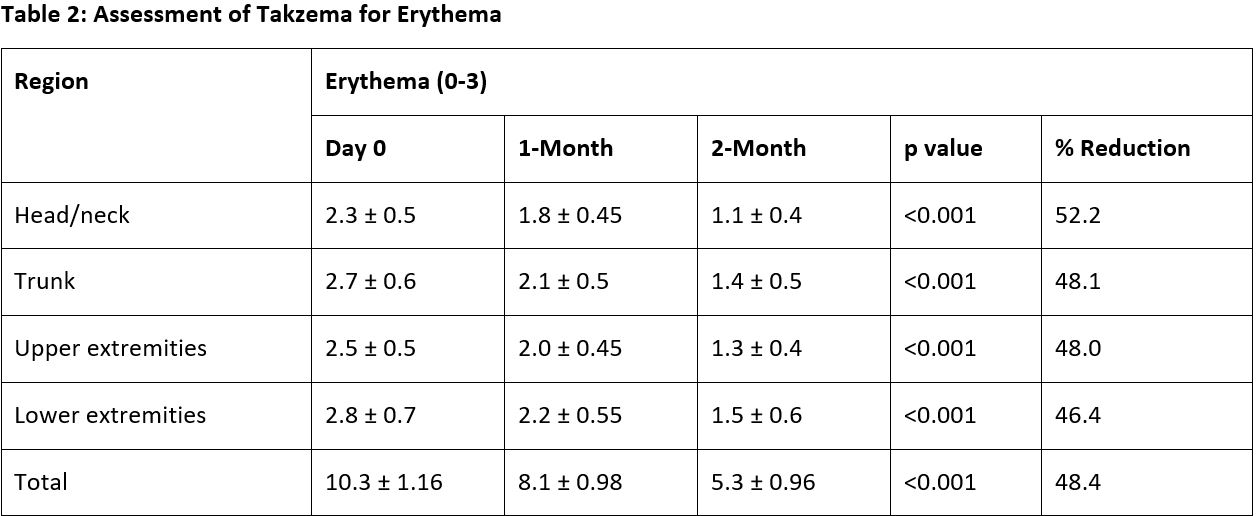
Observation: Over a two-month period, Takzema demonstrated significant improvement across multiple clinical parameters. Erythema scores declined by 48.4% (p < 0.001), with the greatest reduction observed in the head/neck region. Oedema/papulation severity decreased by 63.9% (p = 0.002), with the highest improvement in the trunk (68.4%). Lichenification showed an overall reduction of 47.06% (p = 0.001), with the most significant improvement in the head/neck and upper extremities. The affected area scores also declined by 51.39% (p = 0.002), demonstrating consistent regional improvements.
Result: Takzema Tablets & Ointment demonstrated significant improvements in eczema symptoms after 2 months of treatment, including reductions in erythema, oedema/papulation, lichenification & total area lesions. The results highlight that Takzema tablets & ointment are clinically effective in managing eczema.
Downloads
References
Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1. doi:10.1038/s41572-018-0001-z.
Afshari M, Kolackova M, Rosecka M, Čelakovská J, Krejsek J. Unraveling the skin; a comprehensive review of atopic dermatitis, current understanding, and approaches. Front Immunol. 2024 Mar 4;15:1361005. doi:10.3389/fimmu.2024.1361005.
Paolino A, Alexander H, Broderick C, Flohr C. Non-biologic systemic treatments for atopic dermatitis: current state of the art and future directions. Clin Exp Allergy. 2023;53:495–510. doi:10.1111/cea.14301.
Tumpang MA, Ramli NA, Hussain Z. Phytomedicines are efficient complementary therapies for the treatment of atopic dermatitis: a review of mechanistic insight and recent updates. Curr Drug Targets. 2018;19(6):674–700. doi:10.2174/1389450118666170913162147.
Zawawi NA, Ahmad H, Madatheri R, Fadilah NIM, Maarof M, Fauzi MB. Flavonoids as natural anti-inflammatory agents in the atopic dermatitis treatment. Pharmaceutics. 2025;17:261. doi:10.3390/pharmaceutics17020261.
Hanifin JM, Baghoomian W, Grinich E, Leshem YA, Jacobson M, Simpson EL. The Eczema Area and Severity Index—a practical guide. Dermatitis. 2022 May–Jun;33(3):187–92. doi:10.1097/DER.0000000000000895.
Barta K, Fonacier LS, Hart M, Lio P, Tullos K, Sheary B, Winders TA. Corticosteroid exposure and cumulative effects in patients with eczema: results from a patient survey. Ann Allergy Asthma Immunol. 2023 Jan;130(1):93–99.e10. doi:10.1016/j.anai.2022.09.031.
Devasenapathy N, Asiniwasis R, Netahe R, et al. Cancer risk with topical calcineurin inhibitors, pimecrolimus and tacrolimus, for atopic dermatitis: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2023;7(1):13–25.
Maskey AR, Kopulos D, Kwan M, Reyes N, Figueroa C, Mo X, et al. Berberine inhibits the inflammatory response induced by Staphylococcus aureus isolated from atopic eczema patients via the TNF-α/inflammation/RAGE pathways. Cells. 2024 Oct 1;13(19):1639. doi:10.3390/cells13191639.
Oh JH, Kim SH, Kwon OK, et al. Purpurin suppresses atopic dermatitis via TNF-α/IFN-γ-induced inflammation in HaCaT cells. Int J Immunopathol Pharmacol. 2022;36. doi:10.1177/03946320221111135.
Jayasinghe AMK, Kirindage KGIS, Kim SH, Lee S, Jung K, Shim SY, Ahn G. Protective effect of Curcuma longa L. leaves and pseudostems extract against 1-chloro-2,4-dinitrobenzene-induced atopic dermatitis in BALB/c mice. J Ethnopharmacol. 2025 Feb 10;338(Pt 3):119138. doi:10.1016/j.jep.2024.119138.
Namale N, Tusubira D, Male K, Musyoka AM, Aja PM. Curcuma longa essential oil topically mitigates inflammatory markers in acetone-induced atopic dermatitis in Wistar albino rats. bioRxiv. 2024 Dec 14. doi:10.1101/2024.12.14.628498.
Finberg MJ, Muntingh GL, van Rensburg CEJ. A comparison of the leaf gel extracts of Aloe ferox and Aloe vera in the topical treatment of atopic dermatitis in BALB/c mice. Inflammopharmacol. 2015;23:337–41. doi:10.1007/s10787-015-0251-2.