A Critical Review on Safe Handling of Vegetable Origin Drugs used in Ayurveda from Schedule (E1) of the Drugs and Cosmetics Rules, 1945
DOI:
https://doi.org/10.21760/jaims.10.7.18Keywords:
Visha-Upavisha varga, Poisonous drugs, Schedule (E1), Drugs and Cosmetic Rules 1945Abstract
Introduction: The Drugs and Cosmetics Rules, 1945 were established by the Government of India under the authority of the Drugs and Cosmetics Act, 1940. This Act is designed to ensure that drugs and cosmetics sold in India meet standards for quality, safety, and effectiveness. Schedule (E1) of these rules identifies poisonous substances of plant origin that are used in the Ayurvedic, Siddha and Unani systems of medicine. The focus of the present work is on the safe handling and use of plant origin poisonous drugs used in Ayurvedic system of medicine.
Methodology: A thorough evaluation of literature was done, including the relevant portions of the Drugs and Cosmetics Rules, 1945, authoritative text books of Ayurveda, published research papers in reputed journals.
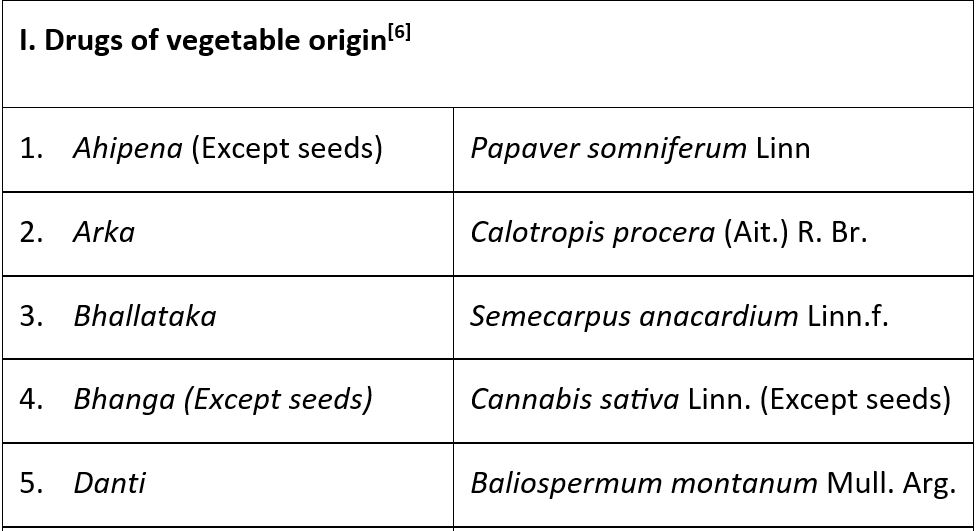
Results: The Drugs and Cosmetics Rules, 1945, which mandates that ASU drugs in which the Ayurvedic system classifies fourteen medications with vegetable origins under the category of poisonous substances. Despite being part of the Visha-Upavisha which is a category of poisonous substances, these medications are not hazardous because Ayurveda recommends a special method of cleansing called Shodhana before utilizing them for therapeutic purposes. Schedule E (1) of the Drugs and Cosmetics Rules, 1945, is associated with Rule 161(2).
Conclusion: Ayurvedic drug manufacturers, dealers, Vaidya and physicians must be aware and focus on the safe manufacturing practices of medicines, rational prescription and safe dosage of medicines and sale of such medicines should be done only under the valid prescription. By ensuring appropriate information to patients regarding the dosage and administration.
Downloads
References
Taiyabi SF. AYUSH: An introduction [Internet]. Ministry of Ayush, Government of India. Available from: https://ayushnext.ayush.gov.in/detail/post/ayush
Ministry of Health and Family Welfare, Government of India. The Drugs and Cosmetics Act and Rules: Ayush drugs [Internet]. p.6. Available from: https://cdn.ayush.gov.in/wpcontent/uploads/2021/09/Drugs-and-Cosmetics%20Act-Rules.pdf
Katiyar CK, Chakrabarty AK, Dubey SK, Pandey PK, Tumulu M, Narwaria A. Enhancing quality of AYUSH products: Strategies and efforts of the Ministry of AYUSH and AYUSH industry. J Res Ayurvedic Sci. 2023;7(Suppl 1):S73–81. Available from: https://www.jrasccras.com
Ministry of Health and Family Welfare, Government of India. Drugs and Cosmetics Act and Rules. 1940; Schedule E(1), Rule 161(2):317.
Ministry of Health and Family Welfare, Government of India. Drugs and Cosmetics Act and Rules. 1945; Schedule E(1), Rule 161(2):300.
Ministry of Health and Family Welfare, Government of India. The Drugs and Cosmetics Act and Rules: Ayush drugs [Internet]. p.300. Available from: https://cdn.ayush.gov.in/wpcontent/uploads/2021/09/Drugs-and-Cosmetics%20Act-Rules.pdf
Ruknuddin, Galib. Recent amendments in D&C Act with reference to Ayurvedic drug industry. Souvenir on Arogyavardhini 2012: JSS Ayurveda Mahavidyalaya, Mysore. [Internet]. Available from: https://www.researchgate.net/publication/264942241
Ministry of Health and Family Welfare, Government of India. The Drugs and Cosmetics Act and Rules: Ayush drugs [Internet]. p.190. Available from: https://cdn.ayush.gov.in/wpcontent/uploads/2021/09/Drugs-and-Cosmetics%20Act-Rules.pdf
Maurya SK, Seth A, Laloo D, Singh NK, Gautam DN, Singh AK. Śodhana: An Ayurvedic process for detoxification and modification of therapeutic activities of poisonous medicinal plants. Ancient Sci Life. 2015 Apr;34(4):188.
Sharma S. Vishopavishadi vijnaniya. In: Shastri K, editor. Rasatarangini. Varanasi: Motilal Banarasidas; 1979. p.647.
Agnivesha. Dantidravanti Kalpam. In: Acharya VJT, editor. Charaka Samhita revised by Charaka and Dridabala with Ayurveda Dipika commentary of Chakrapanidatta. Varanasi: Chaukhambha Orientalia; 2009. p.670.
Agnivesha. Deerghanjeeviteeyam. In: Acharya VJT, editor. Charaka Samhita revised by Charaka and Dridabala with Ayurveda Dipika commentary. Varanasi: Chaukhambha Orientalia; 2009. p.20.
Aparna K, Joshi AJ, Vyas M. Adverse reaction of Parasika Yavani (Hyoscyamus niger Linn): Two case study reports. Ayu. 2015 Apr;36(2):174.
Adhana RK, Chaudhry R. Clinical importance of Upavisha: A therapeutic portrayal of toxic drugs. World J Pharm Res [Internet]. 2019;8(7):1594–1606. Available from: https://wjpr.s3.ap-south-1.amazonaws.com/article_issue/1559304905.pdf
Sumedhan V, Soumya MC, Sinimol TP. Review on Upavishas of clinical significance. J Ayurveda Integr Med Sci [Internet]. 2020 Feb 29;5(1):194–205. Available from: https://jaims.in/jaims/article/view/837
Ministry of Health and Family Welfare, Government of India. The Ayurvedic Pharmacopoeia of India. Part I [Internet]. Vols I–V. Available from: https://dravyagunatvpm.wordpress.com/e-ayupharmacopoeia-of-india
Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 51st ed. Pune: Nirali Prakashan; 2015. p.14.122, 15.19, 15.39, 15.51, 15.54, 15.68.
Central Council for Research in Ayurveda & Siddha. Database on Medicinal Plants used in Ayurveda & Siddha. New Delhi: Dept. of AYUSH, Ministry of Health and Family Welfare; 2007. Vols 1–8.
Indian Council of Medical Research. Quality standards of Indian medicinal plants. New Delhi: ICMR; 2017. Vols 1–14.
Ministry of Health and Family Welfare, Govt. of India. The Ayurvedic Formulary of India. Part I. Appendix II. 2nd revised English ed. 2003. p.361–369.
Ministry of Health and Family Welfare, Govt. of India. The Ayurvedic Pharmacopoeia of India. Part I [Internet]. Vols I–V. Available from: https://dravyagunatvpm.wordpress.com/e-ayupharmacopoeia-of-india
Agnivesha. Deerghanjeeviteeyam. In: Acharya VJT, editor. Charaka Samhita revised by Charaka and Dridabala with Ayurveda Dipika commentary of Chakrapanidatta. Varanasi: Chaukhambha Orientalia; 2009. p.23.
Ilanchezhian R, Roshy JC, Acharya R. Importance of media in Shodhana (purification/processing) of poisonous herbal drugs. Ancient Sci Life. 2010 Oct;30(2):54.
Agnivesha. Atreyabhadrakapyeeyam. In: Acharya VJT, editor. Charaka Samhita revised by Charaka and Dridabala with Ayurveda Dipika commentary of Chakrapanidatta. Varanasi: Chaukhambha Orientalia; 2009. p.138.
Agnivesha. Atreyabhadrakapyeeyam. In: Acharya VJT, editor. Charaka Samhita revised by Charaka and Dridabala with Ayurveda Dipika commentary of Chakrapanidatta. Varanasi: Chaukhambha Orientalia; 2009. p.141.
Vikram ENT, Ilavarasan R, Kamaraj R. Anti-cancer activities of Schedule E1 drugs used in Ayurvedic formulations. J Ayurveda Integr Med. 2022 Apr–Jun;13(2):100545. doi: 10.1016/j.jaim.2022.100545. PMID: 35661925; PMCID: PMC9163510.
Ministry of Health and Family Welfare, Government of India. The Drugs and Cosmetics Act and Rules: Ayush drugs [Internet]. p.190. Available from: https://cdn.ayush.gov.in/wpcontent/uploads/2021/09/Drugs-and-Cosmetics%20Act-Rules.pdf
Ministry of Ayush, Government of India. Public notice [Internet]. 2016 Feb 01. Available from: https://cdn.ayush.gov.in/wpcontent/uploads/2021/03/Public-Notice-in%20English.pdf
Ministry of Consumer Affairs, Government of India. Advisory concerning sale of Ayurveda, Siddha and Unani drugs [Internet]. 2022 Jul 14. Available from: https://affairs.nic.in/sites/default/files/fileuploads/latestnews/ADVISORYConserning%20sale%20of%20Ayurvedic%2C%20Siddha%20and%20Unani%20Drugs.pdf